Acetic Anhydride React With Water
At the carbonyl carbon that belongs to the bigger half.
A general anhydride looks similar this:
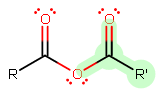
The leaving group is highlighted, assuming
A general dominion of thumb is that the longer the alkyl chain of the
Since we are assuming that
An anhydride literally lacks h2o. (anhydrous = without water.)
Permit's add water.
- H2o attacks the carbonyl carbon. Which one? Pick the one that makes the carboxylate with the
#R'# leave, considering that's what we're assuming volition happen. This is reversible , and actually is slightly favored in the contrary management, so we should add together quite a bit of water to push the equilibrium forward. - Proton transfer via a suitable base. Naturally, the water we added. But this is even so an equilibrium.
- Something has to leave to stabilize this tetrahedral intermediate. We don't want the h2o nosotros just added to gain a proton and then get out. That'southward the contrary reaction. And of course,
#"O"^(two-)# is a much stronger base than#"R"'"OO"^(-)# merely by looking at its charge. So, the#\mathbf(R"'")# carboxylate grouping must leave. At this point information technology's irreversible. - The carboxylate would want a proton. Why? The pKa of a typical carboxylic acid is about
#v# , but the pKa of hydronium is about#-ane.vii# . The weaker acrid exists in its acidic class predominantly. Hence, hydronium gives upwardly its proton nicely.
You tin at present see that two carboxylic acids form.
Challenge: Can y'all describe the mechanism for the aridity of two carboxylic acids into 1 anhydride? Do yous call back it requires heat? How nearly acid?
Acetic Anhydride React With Water,
Source: https://socratic.org/questions/how-do-anhydrides-react-with-water
Posted by: cookgerentow.blogspot.com


0 Response to "Acetic Anhydride React With Water"
Post a Comment